0–1 platform for remote pharmaceutical sampling and provider engagement
The Context
During COVID, pharmaceutical sampling collapsed almost overnight. Providers could not verify eligibility or request samples, and manufacturers had no way to track or fulfill requests. Pfizer asked BCG Digital Ventures to explore whether a fully digital workflow could replace the in person model as a long term operating system for the industry.
We were tasked with building a 0 to 1 sampling platform that unified eligibility, clinical content, compliance checks, and ordering logic into one place providers could trust.
The Problem
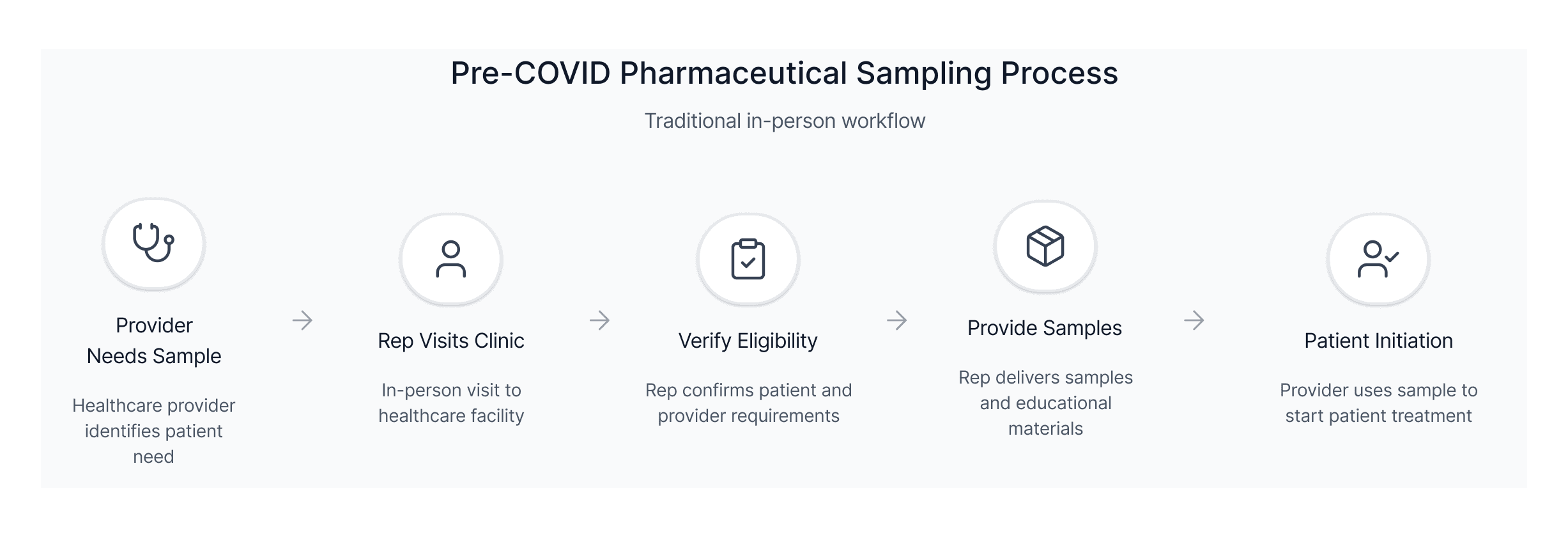
Sampling was never designed to work without a rep. Eligibility checks, compliance steps, clinical guidance, and ordering logic all lived in fragmented offline workflows. To enable digital sampling, we needed to design a unified, compliant workflow that had never existed before.
This workflow revealed how dependent sampling was on in-person rep interactions and why COVID eliminated the primary channel providers relied on.
My Role
As Sr. UX Designer, I led UX across the 0–1 product:
Facilitated discovery with clinicians, pharmacy staff, and pharma leadership
Translated sampling regulations into digital workflows
Created the IA and interaction models
Designed exploration flows and sample-ordering features
Built prototypes for testing and stakeholder alignment
Collaborated with product, engineering, and service design
Integrated feedback from senior stakeholders, including Apple’s former Head of Global Retail
What made the problem hard
Digital sampling had no precedent. We were not digitizing an existing workflow but constructing one under strict constraints:
Rules varied across manufacturers, states, and drug classes, which required a flexible eligibility model
Reps acted as the workflow guide, so tribal knowledge needed to be translated into UI
Clinical content was inconsistent, requiring structure and predictable patterns
Specialist mental models differed, so the UX had to feel universal without losing nuance
Compliance needed to be built in from day one
Timelines were compressed during COVID, and clinics needed a functioning digital path quickly
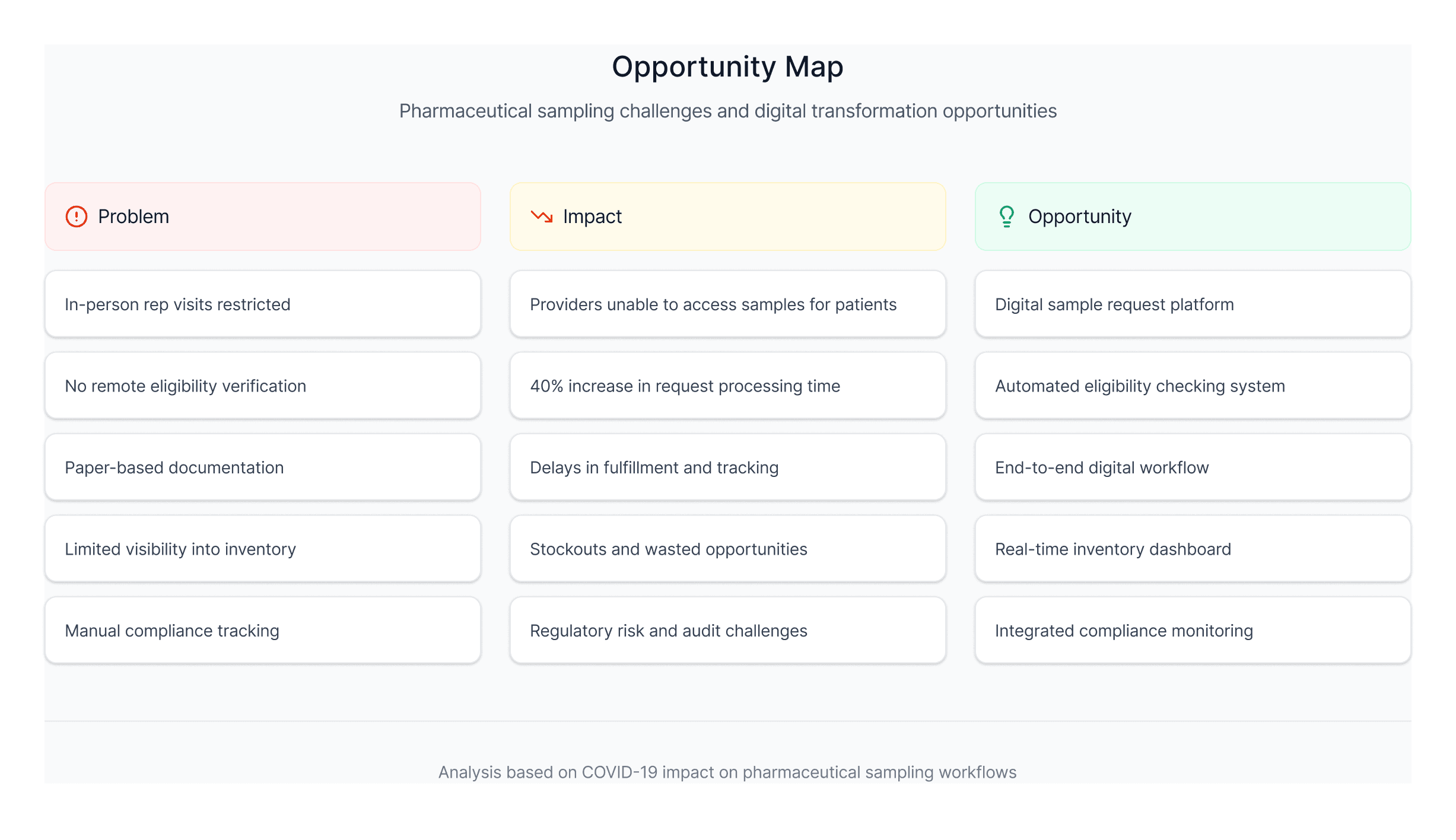
This clarified the real challenge: designing a unified end to end sampling model that preserved clarity and compliance in a setting where none of the steps had ever lived in a single system.

This breakdown made it clear that a digital solution needed to replace rep-led verification, eligibility checks, and sample delivery without adding friction to clinical workflows.
Understanding the remote sampling problem
Through interviews and workflow mapping, I uncovered four barriers preventing a digital transition:
Rep dependency
Eligibility checks, recommendations, and compliance lived in reps’ heads, not systems.Fragmented clinical information
Dosing, guidance, and formulary notes were scattered across PDFs and brand portals.No digital eligibility model
There was no single way to validate licenses, practice settings, or prescribing authority.No digital order infrastructure
Requests, signatures, and tracking required paper based processes.
This revealed the real work ahead: creating a unified model for eligibility, clinical review, and ordering, not just isolated screens.
Defining the digital sampling workflow
Once I understood where traditional workflows were breaking down, the opportunity became clear: build a guided, compliant pathway that could replace the rep led process from start to finish.
Three needs consistently emerged:
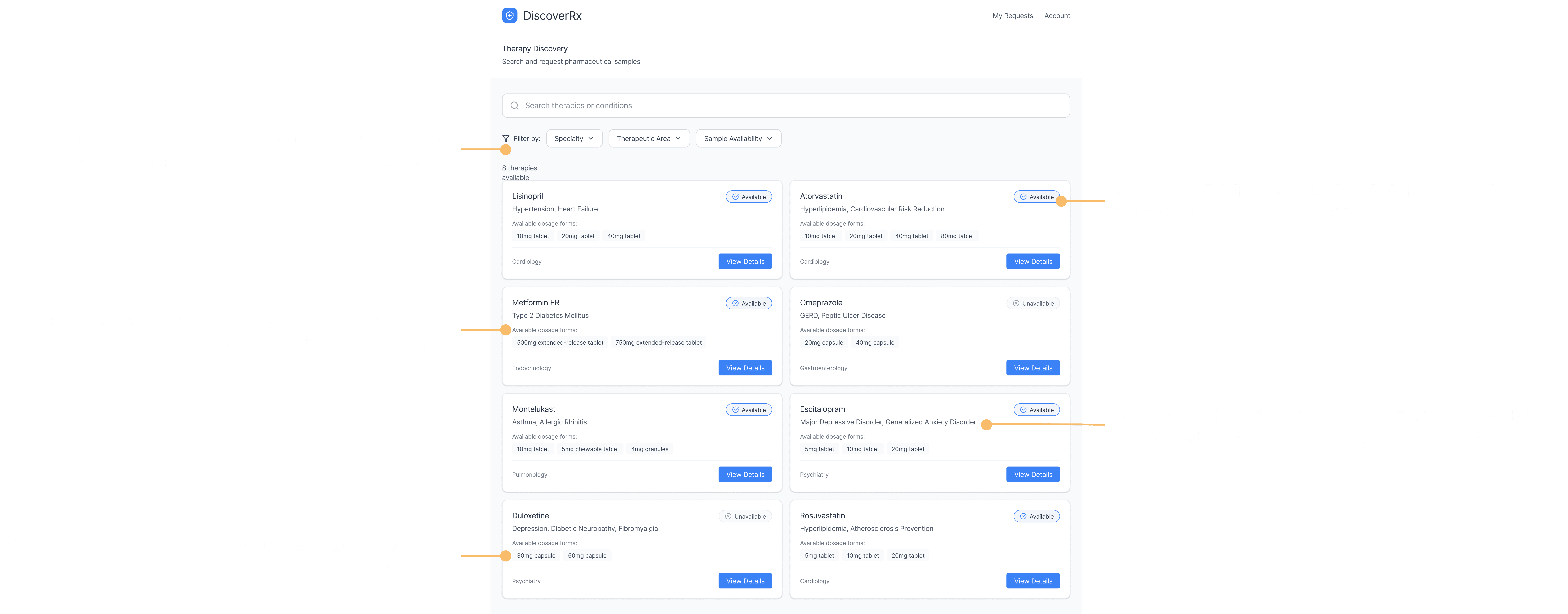
A self service way to request samples anytime
Providers needed a way to explore therapies, review clinical information, and place requests without waiting for rep availability.Automated eligibility and compliance verification
Manufacturers needed digital validation of licenses, practice settings, and program terms to reduce compliance risk.Real time transparency into inventory and order status
Providers needed confidence they were selecting available therapies, and manufacturers needed visibility to streamline fulfillment.
These needs shaped the foundation of the platform: a digital sampling workflow designed to be fast for clinicians and safe for manufacturers.
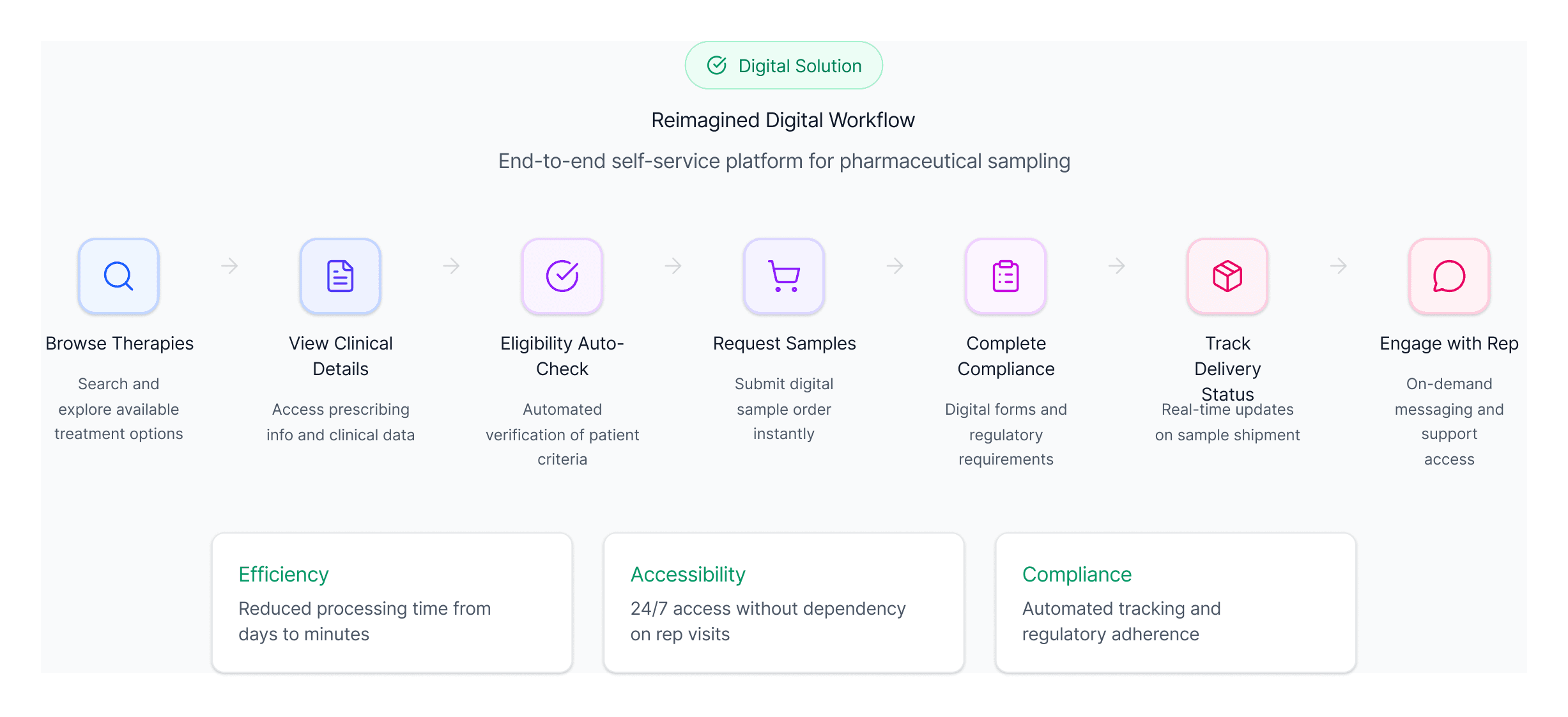
This workflow redefined the sampling process into a fast, compliant, self-service model that preserved speed while reducing operational friction.
Designing the DiscoverRx Product Experience
I translated a fragmented, rep-driven workflow into a unified digital system through three core design moves:
1. Build provider trust through clarity and predictability
Eligibility status surfaced upfront to eliminate uncertainty.
Inventory transparency reduced dead-ends during ordering.
Progressive clinical disclosure kept screens lightweight while maintaining depth.
2. Recreate the value of a rep - without the rep
Embedded guidance replaced real-time rep explanations.
Support entry points (Ask a rep, schedule a call) acknowledged clinics that still rely heavily on field teams.
Workflow maps helped providers understand the journey before initiating a request.
3. Reduce operational and compliance risk for manufacturers
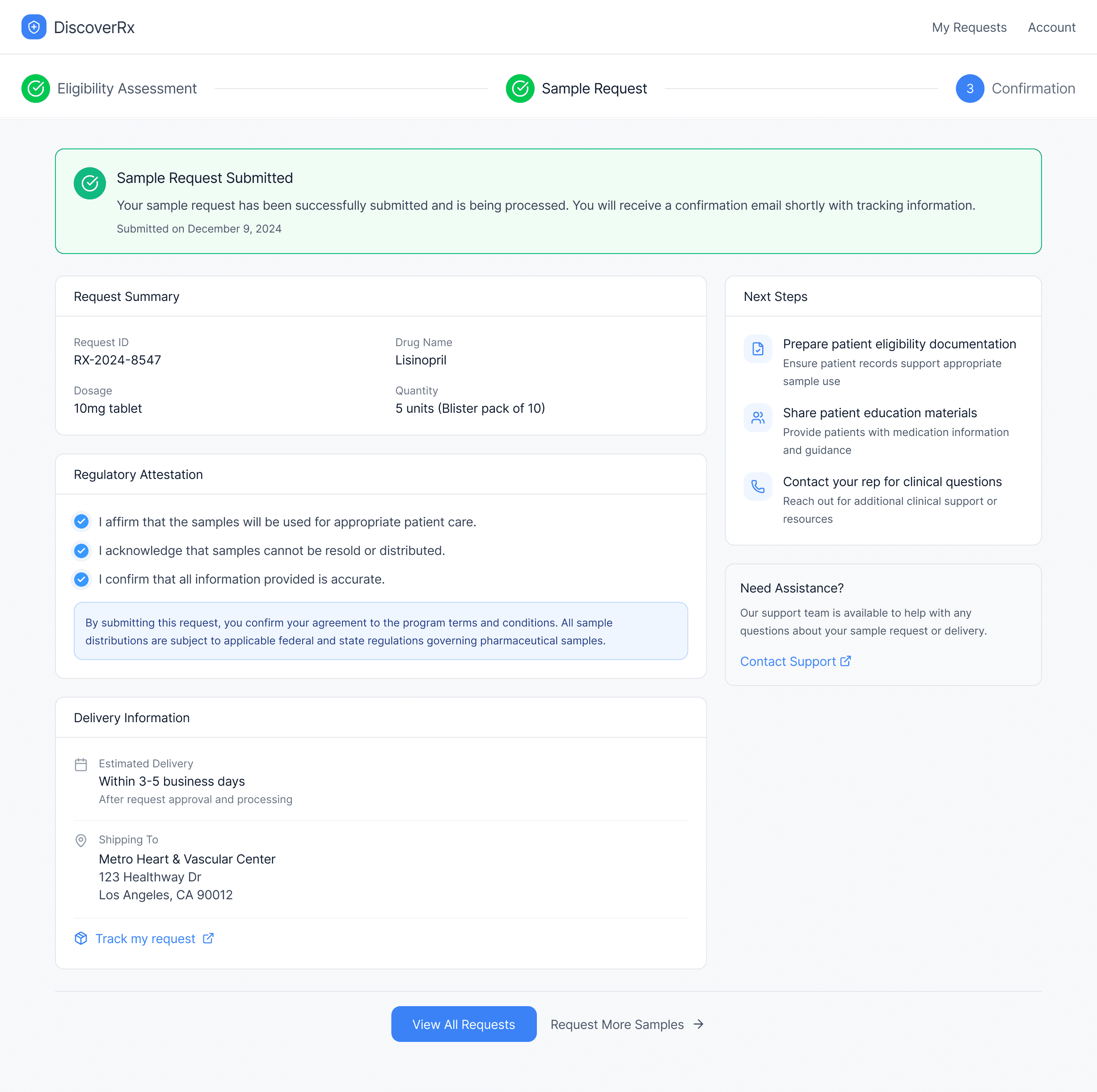
Eligibility criteria were translated into machine-verifiable rules.
Regulatory attestation was integrated directly into the confirmation process.
Consistent data capture ensured audit-ready records from day one.
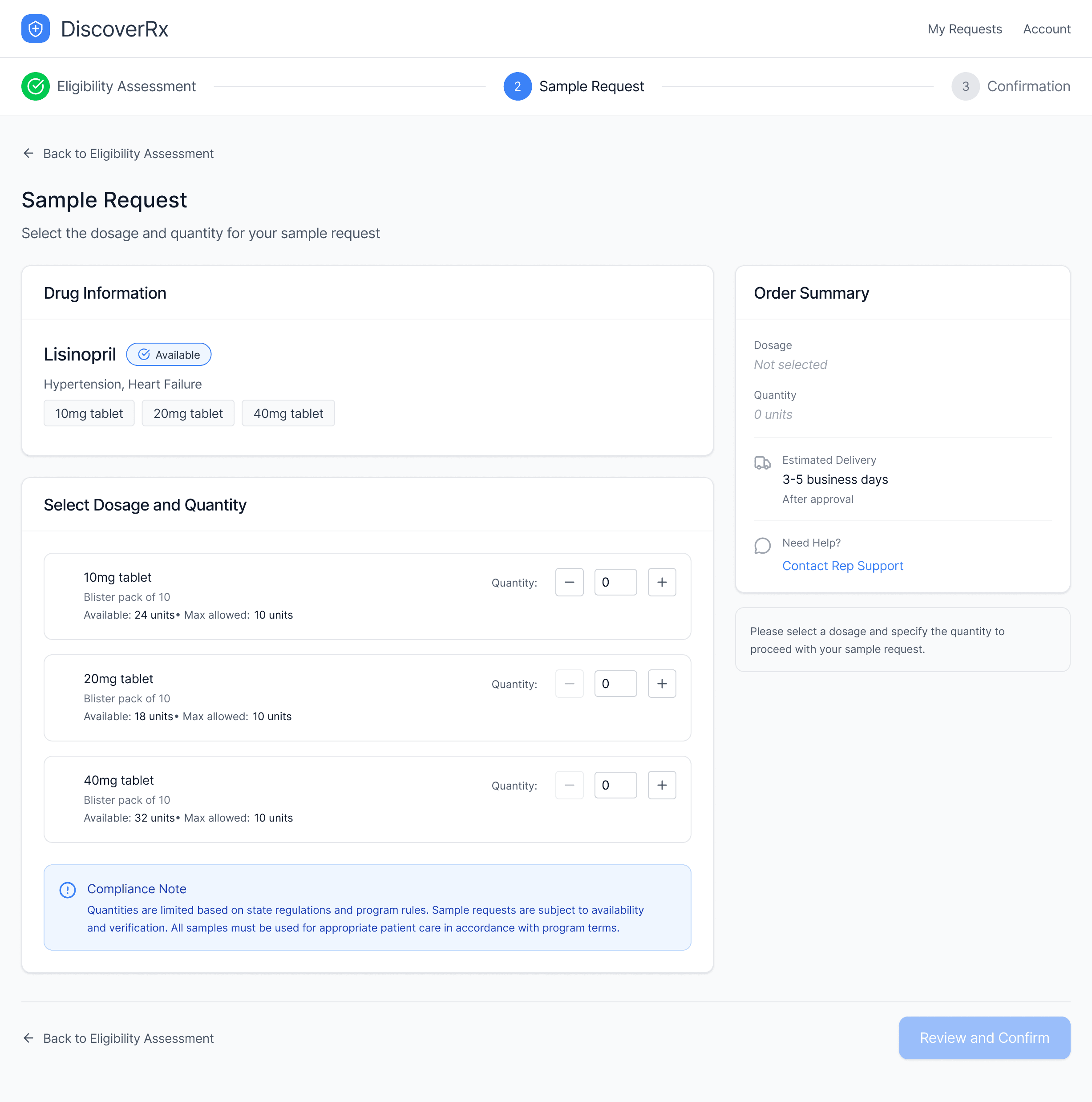
4. Deliver a frictionless ordering experience under uncertainty
Sample selection emphasized speed while enforcing dosing and quantity limits.
Address validation and practice information were surfaced at decision points.
Step indicators kept providers oriented in a new workflow they hadn’t used before.
Research & Workflow Mapping
Once I mapped where the traditional sampling workflow was breaking down, the opportunity became clear: build a unified digital pathway that could replace the rep-driven process end-to-end.
Three needs consistently emerged across providers and manufacturers:
1. A self-service way to request samples anytime
Providers needed a single place to browse therapies, view dosing and clinical information, check eligibility, and place requests without waiting for rep availability.
2. Automated eligibility and compliance verification
Manufacturers required a way to validate licenses, practice settings, prescribing authority, and program terms digitally to reduce compliance risk and eliminate manual reviews.
3. Real-time visibility into inventory and order status
Both sides needed transparency—providers to avoid dead ends, and manufacturers to streamline fulfillment and track demand.
These insights shaped the foundation of the DiscoverRx platform: a fully digital, auditable sampling workflow designed to be faster for clinics and safer for manufacturers.
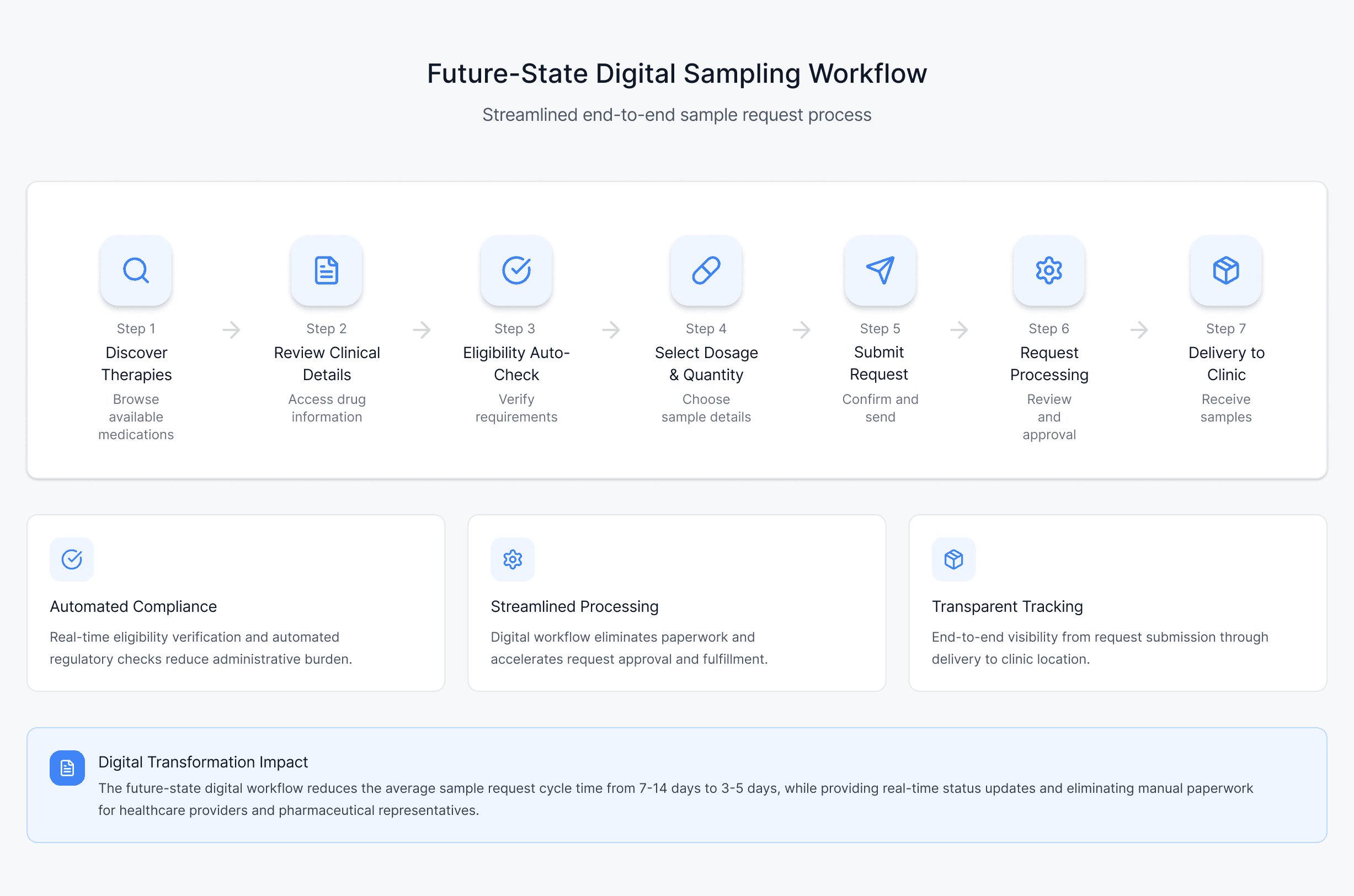
Outcomes & Impact
Based on workflow modeling, prototype testing with providers, and operational analysis, I projected significant improvements across clinical, operational, and experience dimensions.
Provider Impact (Projected)
40–60 percent reduction in time spent confirming eligibility by moving checks upstream.
30 percent decrease in failed or incomplete requests through clearer instructions and compliance gating.
25 percent faster therapy initiation, modeled from fewer rep-related delays and more reliable sample availability.
Strong provider preference for digital workflows observed in early testing.
Operational & Pharma Impact (Projected)
20–30 percent reduction in manual review workload through standardized digital intake.
Increased compliance accuracy by automating eligibility verification and attestations.
Fewer shipment errors based on adding explicit practice-address confirmation prior to submission.
A scalable workflow that could support dozens of therapies without redesign.
Experience Improvements (Observed During Testing)
Reduced a multi-day process to a 3–5 minute guided digital flow.
Improved information findability and reduced cognitive load through consistent card patterns.
Increased clinician confidence and clarity during prototype testing.
What I Learned
Working in a highly regulated healthcare environment taught me the importance of designing for clarity, traceability, and trust. I learned how to balance clinical needs with compliance requirements, and how to align diverse stakeholders toward a unified workflow. Most importantly, I saw how thoughtful information hierarchy and workflow design can meaningfully reduce friction for clinicians and accelerate care for patients.